We are with you every step!
As Contract Research Organization (CRO) we focus on medical devices and medical technology. We offer you individual solutions addressing the entire Clinical Product Lifecycle of your medical device. Benefit from our long-term experience in clinical investigations and evaluations, whether your product already bears CE mark or not.
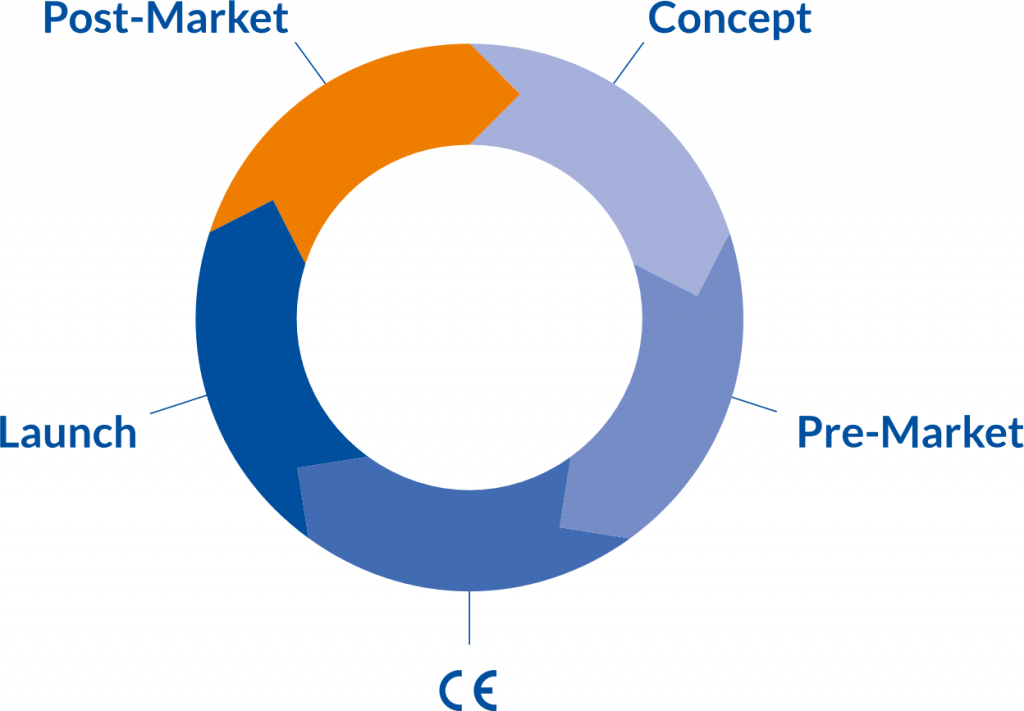
From Start-up to global Player
Our offer is aimed at established medical device manufacturers and ambitious start-ups that want to enter this market. For all of these, the introduction of MDR (Medical Device Regulation, EU Regulation 2017/745) brings high demands on the development and marketing of medical devices.
Our offer is aimed at established medical device manufacturers and ambitious start-ups that want to enter this market. For all of these, the introduction of MDR (Medical Device Regulation, EU Regulation 2017/745) and EU Regulation 2017/746 on IVDR brings high demands on the development and marketing of medical devices and in vitro diagnostic medical devices.
Services
we provide
Clinical Investigations
Need a clinical trial for a medical device in Europe? Would you like to use and improve your medical technology under controlled conditions?
Medical/Regulatory Writing
CERES has extensive medical device experiences within Regulatory, Quality and Clinical Affairs. Devices from class III to I, or implants and active implants are subject of our Medical Writing services.
Post-Market Clinical Follow-up
Does your device already have CE approval and do you need a Post-Market Clinical Follow-up measure?
Consulting
We continuously support you in the developement of a clinical-regulatory strategy, and the optimization of your processes and product Pipeline.
Workshops
In our moderated workshops, we address your company-specific requirements. We pick up your current situation and lead you to a needs-based formulation of your goals.

